Framingham Risk Score Canada
Article Tools
ORIGINAL RESEARCH
Information for this Coronary Heart Disease Risk Calculator comes from the Framingham Heart Study. The results are applicable only for the ages of 30 to 74. Please refer to: Wilson, PW, et. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation 1998 97 (18): 1837-1847. This is known as the modified Framingham Risk Score.3 Step 11 In the “points” column enter the appropriate value according to the patient’s age, HDL-C, total cholesterol, systolic blood pressure, and if they smoke or have diabetes. Calculate the total points. Step 21 Using the total points from Step 1, determine the 10-year CVD risk. (%).
Navigate This Article
Jennifer Gander, MSPH; Xuemei Sui, MD, MPH, PhD; Linda J Hazlett, PhD; Bo Cai, PhD; James R. Hébert, ScD; Steven N. Blair, PED
Suggested citation for this article: Gander J, Sui X, Hazlett LJ, Cai B, Hébert JR, Blair SN. Factors Related to Coronary Heart Disease Risk Among Men: Validation of the Framingham Risk Score. Prev Chronic Dis 2014;11:140045. DOI: http://dx.doi.org/10.5888/pcd11.140045.
PEER REVIEWED
Abstract
Introduction
Coronary heart disease (CHD) remains a leading cause of death in the United States. The Framingham Risk Score (FRS) was developed to help clinicians in determining their patients’ CHD risk. We hypothesize that the FRS will be significantly predictive of CHD events among men in the Aerobics Center Longitudinal Study (ACLS) population.
Methods
Our study consisted of 34,557 men who attended the Cooper Clinic in Dallas, Texas, for a baseline clinical examination from 1972 through 2002. CHD events included self-reported myocardial infarction or revascularization or death due to CHD. During the 12-year follow-up 587 CHD events occurred. Multivariable-adjusted hazard ratios generated from ACLS analysis were compared with the application of FRS to the Framingham Heart Study (FHS).
Results
The ACLS cohort produced similar hazard ratios to the FHS. The adjusted Cox proportional hazard model revealed that men with total cholesterol of 280 mg/dL or greater were 2.21 (95% confidence interval (CI), 1.59–3.09) times more likely to have a CHD event than men with total cholesterol from 160 through 199mg/dL; men with diabetes were 1.63 (95% CI, 1.35–1.98) times more likely to experience a CHD event than men without diabetes.
Conclusion
The FRS significantly predicts CHD events in the ACLS cohort. To the best of our knowledge, this is the first report of a large, single-center cohort study to validate the FRS by using extensive laboratory and clinical measurements.
Introduction
Coronary heart disease (CHD) remains one of the leading causes of death in the United States, accounting for approximately 17% of overall national health care expenditures (1). CHD is the accrual of plaque in the arteries of the heart (2) that supply the blood for maintaining normal cardiac function. The accumulation of plaque narrows the heart’s arteries and reduces blood flow to the heart muscle. The lack of oxygen-rich blood to portions of the heart muscle leads to ischemia of myocardial tissues and consequent alteration of heart function. CHD also can be caused by the deposition of fat beneath the endothelium, reducing the elasticity of arteries (2). This arterial damage is caused by an array of significant risk factors such as hypertension (3), hypercholesterolemia (4), diabetes (5), and smoking (6). However, these risk factors are modifiable through individual and population-level behavior change; through close monitoring of cholesterol, blood glucose, and other risk factors; and by treating any of these risk factors that are above acceptable ranges with medication such as statins or insulin. As a result, many countries have experienced a decrease of CHD incidence in the past 30 years (7).
Several risk scores have been developed to provide guidance to clinicians on their patients’ risk for CHD (8,9). The Framingham Risk Score (FRS) (9,10) is the CHD risk score most widely used by clinicians across the globe (11). The FRS originated from the Framingham Heart Study (FHS), a relatively homogeneous cohort residing in Framingham, Massachusetts (9), and has been applied and validated in a variety of different populations (12,13). However, the study of Kagan et al (13) lacked complete congruency with FRS methodology, and other studies such as those of Lee et al (12) and Fried et al (14) had relatively small sample sizes. A recent publication updated the 1998 FRS and developed a new risk score that predicted an individual’s cardiovascular disease risk instead of the CHD outcome (15). For this study, we chose to investigate CHD outcomes as they comprise the majority of cardiovascular disease events (16).
Our research aims to expand on recent validation studies (17) that used the Aerobics Center Longitudinal Study (ACLS) cohort and the measured outcome of 10-year risk for CHD. ACLS provides a larger cohort to validate FRS than FHS or other previous studies, and FRS has yet to be applied to this cohort. Extensive measures of FRS components and CHD outcomes are available on the more than 40,000 participants (18) in the ACLS cohort. Our objective was to test the hypothesis that the FRS will be significantly predictive of CHD events among men in the ACLS population.
Methods
Study population
ACLS is an observational longitudinal study whose members were patients of the Cooper Clinic, Dallas, Texas, where they received a preventive medical examination and counseling on health behaviors during periodic visits. The Cooper Clinic serves anyone who elects to come for an examination, and patients come from all 50 states. During the patients’ medical examination, they were informed of the ACLS, asked to participate, and if they agreed to participate, they consented to follow-up surveillance. The ACLS protocol was annually reviewed and approved by the Cooper Institute’s institutional review board.
Participants were examined at least once from 1972 through 2002 at the Cooper Clinic. The cohort consisted mostly of patients in the middle and high socioeconomic groups: approximately 80% had college degrees (19). The mean baseline age of the cohort was 42 years (20) and consisted mostly of men (75%) and non-Hispanic whites (>95%).
Although ACLS is not a representative sample of the entire US population, a comparison of median values of specific physiological variables show similarity to representative population data (21).
A large number of women were enrolled in ACLS (n = 11,276); however, women were excluded from this analysis because of the small number of CHD events (n = 45) during the follow-up period. The following inclusion criteria were applied to the ACLS cohort participants for the current study: 1) age at baseline examination from 30 to 74 years, 2) complete data for outcome and predictor variables, and 3) free of CHD diagnosis or cancer diagnosis at baseline. To control for any unmeasured confounders that may have caused early drop-out, men with less than 1 year of follow-up were excluded from the study’s cohort (Figure 1).
Figure 1. Study flow and Aerobic Center Longitudinal Study inclusion criteria depicting final sample size and coronary heart disease event frequency. Men with complete Framingham Risk Score data and body mass index ≥ 18.5 kg/m2 were included in the analysis. Abbreviations: FRS, Framingham Heart Study; CHD, coronary heart disease; BMI, body mass index. [A tabular version of this figure is also available.]
Clinical examination
Trained technicians followed standardized protocols in conducting each measurement. The baseline clinical examination included a personal and family medical history, anthropometric measurements, a 12-hour fasting blood chemistry including glucose and cholesterol measurements, electrocardiogram, blood pressure assessment, and a maximal exercise test (21,22).
CHD was the primary end point being investigated. CHD was defined as the self-report of myocardial infarction or revascularization (including, bypass, coronary balloon, angioplasty, or stent) or death due to CHD. Participants reported their history of infarction or revascularization and incident date through a mail-back questionnaire administered in 1982, 1986, 1990, 1995, 1999, and 2004. Deaths among study participants were identified from the National Center for Health Statistic’s National Death Index. International Classification of Disease (ICD), Ninth Revision, codes 410.0–414.0 and Tenth Revision, codes I20–I25, were used to identify CHD as the primary cause of death. According to the FRS follow-up time definition, the maximal follow-up time was 12 years. The 12-year follow-up was used in the regression and survival analysis and then adapted to provide 10-year CHD incidence estimates.
The covariates considered for analyses in the ACLS population mimicked the variables included in the recently updated FRS (10). Hypertension was divided into 4 categories according to systolic blood pressure and diastolic blood pressure. Systolic blood pressure was categorized into 4 levels: <130 mm Hg, 130–139 mm Hg, 140–159 mm Hg, or ≥160 mm Hg, and diastolic blood pressure was categorized into 4 levels: <85 mm Hg, 85–89 mm Hg, 90–99 mm Hg, and ≥ 100 mm Hg. When an participant’s blood pressure fell into different categories for systolic and diastolic blood pressure, the higher category was chosen for categorization. For example, if a participant’s blood pressure was 130/80 (systolic blood pressure/diasystolic blood pressusre), the corresponding categories for systolic blood pressure would be 2, and the diastolic blood pressure category would be 1. To determine the hypertension category, the higher classification would be chosen and the hypertension categorization would be 2 in this example. Hypertension definition was made without regard to a participant’s use of antihypertensive medications. The definition of hypertension parallels the FRS definition (10).
Total cholesterol was grouped into four levels: <200 mg/dL, 200–239 mg/dL, 240–279 mg/dL, and ≥ 280 mg/dL. High-density lipoprotein was categorized as: <35 mg/dL, 35–59 mg/dL, and ≥ 60 mg/dL. A 12-hour fasting glucose >140 mg/dL classified an individual as having diabetes. Smoking status was dichotomized as current smoker or nonsmoker. All categorizations and definitions were analogous to FRS covariate groupings (10).
Statistical analysis
Descriptive statistics were generated to compare the ACLS population with the FRS population. Men in each cohort were compared on mean age; percentage within each category in hypertension, total cholesterol, and HDL; percentage with diabetes, and percentage of current smokers. Univariate Cox Proportional Hazard models were performed for the CHD events and each covariate to determine each characteristic’s predictive power. Cox Survival analyses were conducted to determine the 10-year CHD risk for the ACLS male population. The fully adjusted Cox Proportional Hazard model included age, blood pressure, total cholesterol, high density lipoprotein cholesterol, diabetes diagnosis, and smoking status.
Predictive accuracy was determined through the concordance statistic (C statistic) associated with the receiver operating characteristic (ROC) curve. The ROC curve measures the discrimination power of these diagnostic markers for the CHD outcome. The Hosmer-Lemeshow statistic is used to assess calibration and is a χ2 test calculated by sorting the sample by estimated probability of success (23). The higher the C statistic, the better the prediction. A limitation of the Hosmer-Lemeshow test is that it is not recommended for sample sizes larger than 25,000. A sensitivity analysis was performed following the recommendations of Paul et al (23), and the ACLS sample (n = 34,557) and a smaller 10,000 sample cohort were randomly selected. To satisfy this limitation, the Hosmer-Lemeshow test was performed on a randomly selected cohort (n = 10,000), and P value of P > .05 represents no significant difference between predicted and observed events. All analyses were performed using SAS version 9.3.
Results
During the 12-year follow-up period (284,572 person-years of exposure), 587 men had a CHD event. The incidence rate was 20 per 10,000 person-years. The ACLS cohort had approximately 32,000 more participants (Table 1) than the FHS, and participants were, on average, younger (P < .001). FHS had a higher proportion of people with diabetes (5.0%) and smokers (40.0%) than the ACLS cohort, which had 1.5% and 17.0%, respectively (P < .001) (Table 1).
When the ACLS cohort is stratified by CHD status, men who experienced a CHD event during the 12-year follow-up were significantly different on all predictor variables; that is, they were older, had higher blood pressure, and were in the top 2 categories for high-density lipoprotein cholesterol. Among those men who experienced CHD during follow-up, 4.6% had diabetes and 23.3% were smokers compared with 1.47% (P < .001) with diabetes and 16.8% current smokers (P < .001) who did not experience CHD (Table 2).
The covariates based on the FRS were all significant when applied to the men in ACLS (Table 3). The hazard ratios (HRs) reported from FHS by D’Agostino et al (2001) (17) were similar to the ACLS fully adjusted HRs. The fully adjusted HRs show that men with Stage I hypertension (HR = 1.41; 95% confidence interval [CI], 1.16–1.72) have significantly higher risk of CHD than men with optimal or normal blood pressure. Men with total cholesterol at or greater than 280mg/dL were more than twice (HR, 2.21; 95% CI, 1.59–3.09) as likely to have a CHD event than men with total cholesterol between 160 and 199mg/dL. Men with diabetes were 1.82 (95% CI, 1.23–2.70) times more likely to experience a CHD event than men without diabetes. Current smokers also had a significantly higher risk (HR, 1.63; 95% CI, 1.35–1.98) for CHD than nonsmokers during the 12-year follow-up.
The C statistic (area under the curve) obtained from the receiver operating characteristic (ROC) curve was 0.7697 (95% CI, 0.7523–0.7871) (Figure 2). The Hosmer-Lemeshow test reported no significant lack of fit for the model ( P = .88), and we failed to reject the null hypothesis that states there is no significant difference between the predicted and observed values of the outcome variable.
Figure 2. Receiver operating characteristic curve representing the predictive ability of the Framingham Risk Score applied to the Aerobics Center Longitudinal Study cohort after a 12-year follow-up. The Hosmer-Lemeshow C statistic is represented by the Area Under the Curve (C = 0.7697, 95% confidence interval, 0.7523–0.7871). [A tabular version of this figure is also available.]
Discussion
The FRS significantly predicts CHD events occurring during the 12-year follow-up in the ACLS, which was a much larger study than the original FHS. In addition to our main finding, we also found that age, blood pressure, total cholesterol, high-density lipoprotein cholesterol, diabetes diagnosis, and smoking status were associated with CHD events. The relative risks were congruent with the those reported from the FHS (17) and previous studies (12,13).
Elevated blood pressure creates more strain for the heart, which can cause stiffness of the heart muscle (2) or create microscopic tears in the walls that may develop into scar tissue (2). Myocardial ischemia is common among patients with hypertension (3,5), and reports from the FHS showed that hypertension was the primary cause of congestive heart failure in 35% of cases (24). Men with diabetes are also at increased risk for CHD (25), and additional research shows that people with both diabetes and hypertension have a higher incidence of heart disease than people with diabetes or hypertension alone (5).
Doyle et al published the findings of a study that examined the association between smoking and CHD (26) in two prospective studies: The FHS and the cohort from the Albany, New York, civil service study, with a combined study population of over 1,800 men without CHD (26). The Doyle et al study concluded that men with elevated systolic blood pressure and elevated total cholesterol who smoked were at a 1.8 (P < .05) times higher risk of premature mortality than men with elevated systolic blood pressure and elevated total cholesterol who did not smoke (26). Our findings are also in line with the Physicians’ Health Study, which reported significant effects of HDL cholesterol and total cholesterol on CHD (17).
Other researchers investigated FRS’s predictability in various populations. The Honolulu Heart Study began in 1965 with the overall goal of standardizing cardiovascular examinations (13). The cohort comprised Japanese American men born between 1900 and 1919 whose data were updated with information from their World War II Selective Service files; the final population comprised approximately 8,000 men free of CHD on baseline examination at study initiation (13). Cigarette smoking, cholesterol levels, blood pressure, sum of arm and back skinfold measurements , and uric acid levels were significant predictors of CHD; however, glucose intolerance showed no significant relationship to CHD. The lack of congruency in the significant results between the Honolulu Heart Study, FHS, and ACLS may be due to the Honolulu Heart Study population being at low risk of CHD (ie, CHD incidence observed in the Honolulu Study was about half that of the FHS).
To the best of our knowledge, this is the first large, single-center, prospective cohort to validate the FRS with the same level of precision as that of the FHS. The present study expands on previous research through the improvement of internal validity by using objectively measured clinical data.
A limitation of the ACLS cohort (similar to an FHS limitation) is the homogeneity of the study population’s sociodemographic factors. This limitation was explored through comparison analysis between ACLS and 2 large population-based cohorts; ACLS results were found to be similar to the results of the Lipid Research Clinics Prevalence Survey and the Canada Fitness Survey (27). It should be noted that ACLS homogeneity may be a strength because it improves internal validity by controlling for potential demographic confounders such as education, socioeconomic status, and race/ethnicity; however, generalizations must be made cautiously, and future research should be conducted on more diverse populations. Unlike the FHS finding, the ACLS found that having stage II–IV hypertension was not significantly associated with CHD, which may be due to the small proportion (4.93%) of the ACLS’s cohort who were in this group.
Although CHD remains one of the leading causes of death in the United States, the prevalence of CHD has decreased since 2004 (28), a reduction that can be largely attributed to better medical treatment and improvement in CHD risk profiles. The FRS was developed to assist clinicians in estimating their patients’ absolute risk for CHD (17). This study further evaluates FRS performance in the larger ACLS cohort and strictly followed the FHS methodology, which does not control for other CHD risk factors such self-rated health status (29), family history of CHD (30), and cardiorespiratory fitness (18). Future research should focus on expanding the FRS to include other modifiable risk factors. Community interventions and education programs should continue to target these CHD risk factors to further the prevention of heart disease.
Acknowledgments
Preparation of this article was supported by National Institutes of Health, grants AG06945, HL62508, and DK088195. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management. The authors report no conflicts of interest.
Author Information
Corresponding Author: Xuemei Sui, University of South Carolina, 921 Assembly Street, Rm 226, Columbia, SC 29208. Telephone: 803-777-3881. E-mail: msui@mailbox.sc.edu.
Author Affiliations: Jennifer Gander, Linda J Hazlett, Bo Cai, James R. Hébert, Steven N. Blair, University of South Carolina, Columbia, South Carolina.
References
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics — 2010 update: a report from the American Heart Association. Circulation 2010;121(7):e46–e215. Erratum in: Circulation. 2010 Mar 30;121(12):e260. Stafford, Randall [corrected to Roger, Véronique L]. Circulation. 2011 Oct 18;124(16):e425. CrossRefPubMed
- Coronary Heart Disease. American Heart Association; 2013. http://www.heart.org/HEARTORG/Conditions/More/MyHeartandStrokeNews/Coronary-Artery-Disease — The-ABCs-of-CAD_UCM_436416_Article.jsp. Accessed December 20, 2013.
- Strauer B-E. Myocardial oxygen consumption in chronic heart disease: role of wall stress, hypertrophy, and coronary reserve. Am J Cardiol 1979;44(4):730–40. CrossRefPubMed
- Wijeysundera HC, Machado M, Farahati F, Wang X, Witteman W, van der Velde G, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994-2005. JAMA 2010;303(18):1841–7. CrossRefPubMed
- Grossman E, Messerli FH. Diabetic and hypertensive heart disease. Ann Intern Med 1996;125(4):304–10. CrossRefPubMed
- Scheidt S. Changing mortality from coronary heart disease among smokers and nonsmokers over a 20-year interval. Prev Med 1997;26(4):441–6. CrossRefPubMed
- Bennett K, Kabir Z, Unal B, Shelley E, Critchley J, Perry I, et al. Explaining the recent decrease in coronary heart disease mortality rates in Ireland, 1985–2000. J Epidemiol Community Health 2006;60(4):322–7. CrossRefPubMed
- Assmann G, Schulte H. The Prospective Cardiovascular Münster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am Heart J 1988;116(6 Pt 2):1713–24. CrossRefPubMed
- Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol 1976;38(1):46–51. CrossRefPubMed
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837–47. CrossRefPubMed
- Sposito AC, Ramires JA, Jukema JW, Molina JC, da Silva PM, Ghadanfar MM, et al. . Physicians' attitudes and adherence to use of risk scores for primary prevention of cardiovascular disease: cross-sectional survey in three world regions. Curr Med Res Opin 2009;25(5):1171–8. CrossRefPubMed
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Le N-A, Oopik AJ, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol 1990;132(6):1141–55. PubMed
- Kagan A, Gordon T, Rhoads GG, Schiffman JC. Some factors related to coronary heart disease incidence in Honolulu Japanese men: the Honolulu Heart Study. Int J Epidemiol 1975;4(4):271–9. CrossRefPubMed
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The cardiovascular health study: design and rationale. Ann Epidemiol 1991;1(3):263–76. CrossRefPubMed
- D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care. Circulation 2008;117(6):743–53. CrossRefPubMed
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 2013;127(1):e6. CrossRefPubMed
- D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores. JAMA 2001;286(2):180–7. CrossRefPubMed
- Blair SN, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality of healthy men and women. JAMA 1989;262(17):2395–401. CrossRefPubMed
- Kampert JB, Blair SN, Barlow CE, Kohl HW. Physical activity, physical fitness, and all-cause and cancer mortality: a prospective study of men and women. Ann Epidemiol 1996;6(5):452–7. CrossRefPubMed
- Sui X, Hooker SP, Lee I-M, Church TS, Colabianchi N, Lee C-D, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care 2008;31(3):550–5. CrossRefPubMed
- Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol 1989;129(6):1145–56. PubMed
- Blair SN, Kampert JB, Kohl HW 3d, Barlow CE, Macera CA, Paffenbarger RS Jr, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996;276(3):205–10. CrossRefPubMed
- Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer–Lemeshow goodness of fit test in large data sets. Stat Med 2013;32(1):67–80. CrossRefPubMed
- Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med 1972;287(16):781–7 CrossRefPubMed
- Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339(4):229–34. CrossRefPubMed
- Doyle JT, Dawber TR, Kannel WB, Kinch SH, Kahn HA. The relationship of cigarette smoking to coronary heart disease. JAMA 1964;190(10):886–90. CrossRefPubMed
- Sui X. Longitudinal analyses of physical activity and cardiorespiratory fitness on adiposity and glucose levels. ProQuest Dissertations and Theses. 2012;126.
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation 2010;121(4):586–613. CrossRefPubMed
- Gander J, Lee D-c, Sui X, Hébert JR, Hooker SP, Blair SN. Self-rated health status and cardiorespiratory fitness as predictors of mortality in men. Br J Sports Med 2011;45(14):1095–100. CrossRefPubMed
- Brown WM, Beck SR, Lange EM, Davis CC, Kay CM, Langefeld CD, et al. Age-stratified heritability estimation in the Framingham Heart Study families. BMC Genet 2003;4(Suppl 1):S32. CrossRefPubMed
Tables
Table 1. Comparison Between Demographic Characteristics of Men Free of Coronary Vascular Disease at Baseline in the Framingham Heart Study (FHS) and the Aerobics Center Longitudinal Study (ACLS)a
| Risk Factor | Study Comparisonb | |
|---|---|---|
| FHSc (n = 2,439) | ACLS (n = 34,557) | |
| Age range, y | 30–74 | 30–74 |
| Mean age, y | 48.30 | 44.82 |
| Blood pressure, (mm Hg) | ||
| Optimal and normal (SBP <130, DBP <85) | 44.00 | 59.85 |
| High normal (SBP 130–139, DBP 85–89) | 20.00 | 16.24 |
| Stage I hypertension (SBP 140–159, DBP 90–99) | 23.00 | 18.98 |
| Stage II–IV hypertension (SBP ≥160, DBP ≥100) | 13.00 | 4.93 |
| Total cholesterol (mg/dL) | ||
| <160 | 7.00 | 9.34 |
| 160-199 | 31.00 | 34.36 |
| 200-239 | 39.00 | 36.67 |
| 240-279 | 17.00 | 15.10 |
| ≥280 | 6.00 | 4.53 |
| High-density lipoprotein cholesterol (mg/dL) | ||
| <35 | 19.00 | 16.24 |
| 35-59 | 70.00 | 70.97 |
| ≥60 | 11.00 | 12.79 |
| Diabetes | 5.00 | 1.52 |
| Current smoker | 40.00 | 16.95 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
a Numbers are expressed as percentages unless otherwise stated.
b Independent t test was used to determine statistically significant difference in age between FHS and ACLS participants; proportion test calculated the statistical difference for each level of blood pressure, total cholesterol, high-density lipoprotein cholesterol, diabetes, and current smoking between FHS and ACLS participants. All proportion tests were significant with a P value < .001.
c FHS, Framingham Risk Score descriptive statistics referenced from D'Agostina et al (17).
Table 2. Comparison Between Demographic Characteristics of Men With and Without a Coronary Heart Disease (CHD) Event in the Aerobic Center Longitudinal Study (ACLS)a
| RISK FACTOR | CHD Event Comparison within ACLSb | |
|---|---|---|
| No CHD (n = 33,970) | With CHD (n = 587) | |
| Median follow-up time (IQR) | 10.94 (3.82, 12.00) | 5.66 (2.94, 8.93) |
| Age, range (years) | 30-74 | 30-73 |
| Mean age, y | 44.70 | 51.91 |
| Blood pressure, (mm Hg) | ||
| Optimal and normal (SBP <130, DBP <85) | 60.06 | 47.53 |
| High normal (SBP 130–139, DBP 85–89) | 16.18 | 19.76 |
| Stage I hypertension (SBP 140–159, DBP 90–99) | 18.85 | 26.41 |
| Stage II-IV hypertension (SBP ≥160, DBP ≥100) | 4.90 | 6.30 |
| Total cholesterol (mg/dL) | ||
| <160 | 9.44 | 3.92 |
| 160–199 | 34.62 | 19.59 |
| 200–239 | 36.60 | 40.37 |
| 240–279 | 14.88 | 27.60 |
| ≥280 | 4.46 | 8.52 |
| High-density lipoprotein cholesterol (mg/dL) | ||
| <35 | 16.08 | 25.55 |
| 35–59 | 71.05 | 66.44 |
| ≥60 | 12.88 | 8.01 |
| Diabetes | 1.47 | 4.60 |
| Current smoker | 16.84 | 23.34 |
Abbreviations: IQR = interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure.
a The numbers are percentages unless otherwise stated.
b χ2 test was performed to calculate statistical difference between the group with and without CHD. All comparisons were significant at P < 0.05.
Table 3. Comparison Between Hazard Ratios for Coronary Heart Disease (CHD) Events for the Framingham Heart Study (FHS) Cohort and the Aerobics Center Longitudinal Study (ACLS) Cohort
| Risk Factor | FHSa | ACLS 12y Follow-up | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Fully Adjustedb | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Age, y | 1.05 | 1.04–1.06 | 1.09 | 1.08–1.10 | 1.09 | 1.08–1.10 |
| Blood pressure, mm Hg | ||||||
| Optimal and normal (SBP <130, DBP <85) | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| High normal (SBP 130–139, DBP 85–89 | 1.31 | 0.98–1.76 | 1.66 | 1.33–2.06 | 1.33 | 1.07–1.66 |
| Stage I hypertension (SBP 140–159, DBP 90–99) | 1.67 | 1.28–2.18 | 1.95 | 1.60–2.38 | 1.41 | 1.16–1.72 |
| Stage II-IV hypertension (SBP ≥160, DBP ≥100) | 1.84 | 1.37–2.06 | 1.94 | 1.37–2.73 | 1.23 | 0.87–1.74 |
| Total cholesterol (mg/dL) | ||||||
| <160 | 0.69 | 0.31–1.52 | 0.77 | 0.49–1.21 | 0.82 | 0.52–1.28 |
| 160–199 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] | |||
| 200–239 | 1.77 | 1.25–2.50 | 1.85 | 1.48–2.31 | 1.59 | 1.27–1.99 |
| 240–279 | 2.10 | 1.43–3.10 | 2.90 | 2.28–3.68 | 2.37 | 1.86–3.01 |
| ≥280 | 2.29 | 1.39–3.76 | 2.74 | 1.97–3.83 | 2.21 | 1.59–3.09 |
| High density lipoprotein cholesterol (mg/dL) | ||||||
| <35 | 1.47 | 1.16–1.86 | 1.59 | 1.32–1.92 | 1.60 | 1.32–1.94 |
| 35–59 | 1.00 [Reference] | 1.00 [Reference] | 1.00[Reference] | |||
| ≥60 | 0.56 | 0.37–0.83 | 0.66 | 0.49–0.90 | 0.60 | 0.44–0.81 |
| Diabetes | 1.50 | 1.06–2.13 | 3.45 | 2.34–5.07 | 1.82 | 1.23–2.70 |
| Smoking status | 1.68 | 1.37–2.06 | 1.60 | 1.32–1.93 | 1.63 | 1.35–1.98 |
Abbreviations: HR, hazard ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure.
a Framingham Heart Study hazard ratios are from Wilson et al (10).
b Fully adjusted model included age, blood pressure, total cholesterol, high density lipoprotein levels, diabetes diagnosis, and smoking status.
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions.
The Framingham Risk Score is a gender-specific algorithm used to estimate the 10-year cardiovascular risk of an individual. The Framingham Risk Score was first developed based on data obtained from the Framingham Heart Study, to estimate the 10-year risk of developing coronary heart disease.[1] In order to assess the 10-year cardiovascular disease risk, cerebrovascular events, peripheral artery disease and heart failure were subsequently added as disease outcomes for the 2008 Framingham Risk Score, on top of coronary heart disease.[2]
- 7Versions
- 10Scoring
Cardiovascular Risk Scoring systems[edit]
The Framingham Risk Score is one of a number of scoring systems used to determine an individual's chances of developing cardiovascular disease. A number of these scoring systems are available online.[3][4] Cardiovascular risk scoring systems give an estimate of the probability that a person will develop cardiovascular disease within a specified amount of time, usually 10 to 30 years.[5]
Because they give an indication of the risk of developing cardiovascular disease, they also indicate who is most likely to benefit from prevention. For this reason, cardiovascular risk scores are used to determine who should be offered preventive drugs such as drugs to lower blood pressure and drugs to lower cholesterol levels.
For example, nearly 30% of coronary heart disease (CHD) events in both men and women were singularly attributable to blood pressure levels that exceeded high normal (≥130/85), showing that blood pressure management and monitoring is paramount both to cardiovascular health and prediction of outcomes.[6]
Usefulness[edit]
Because risk scores such as the Framingham Risk Score give an indication of the likely benefits of prevention, they are useful for both the individual patient and for the clinician in helping decide whether lifestyle modification and preventive medical treatment, and for patient education, by identifying men and women at increased risk for future cardiovascular events.[7]
Coronary heart disease (CHD) risk at 10 years in percent can be calculated with the help of the Framingham Risk Score. Individuals with low risk have 10% or less CHD risk at 10 years, with intermediate risk 10-20%, and with high risk 20% or more. However it should be remembered that these categorisations are arbitrary.
A more useful metric is to consider the effects of treatment. If a group of 100 persons all have a 20% ten-year risk of cardiovascular disease it means that we should expect that 20 of these 100 individuals will develop cardiovascular disease (coronary heart disease or stroke) in the next 10 years and eighty of them will not develop cardiovascular disease in the next 10 years.
If they were to take a combination of treatments (for example drugs to lower cholesterol levels plus drugs to lower blood pressure) that reduced their risk of cardiovascular disease by half it means that 10 of these 100 individuals should be expected to develop cardiovascular disease in the next 10 years and 90 of them should not be expected to develop cardiovascular disease. If that was the case then 10 of these individuals would have avoided cardiovascular disease by taking treatment for 10 years; 10 would get cardiovascular disease whether or not they took treatment; and 80 would not have got cardiovascular disease whether or not they took treatment.
Despite their widespread popularity, randomised trials assessing the impact of using cardiovascular disease risk scores show limited impact on patient outcomes. Although there is good evidence that targeting individuals with high total CVD risk is the most efficient way to reduce CVD-related morbidity and mortality, to date trials assessing the usefulness of risk scores at helping clinicians target high risk patients show limited benefit.[8]
Issues Raised by Cardiovascular Risk Prediction[edit]
It is important to recognize that the strongest predictor of cardiovascular risk in any risk equation is age. Almost all persons aged 70 and over are at >20% ten year cardiovascular risk and almost nobody aged under 40 is at >20% ten year cardiovascular risk. Since those who benefit most from treatment are those at highest risk, this means that treatment of patients with raised blood pressure and raised cholesterol levels in their thirties benefits very few, whereas treatment of patients with 'normal' blood pressure and 'normal' cholesterol levels in their seventies benefits many. This casts doubt on the wisdom of categorizing individuals as having high blood pressure or raised cholesterol and treating these individual risk factors without a consideration of both their overall risk of cardiovascular disease and of the probability that they will benefit.
Background[edit]
Cardiovascular disease is common in the general population, affecting the majority of adults. It includes:
- Coronary heart disease (CHD): Myocardial infarction (MI), angina pectoris, heart failure (HF), and coronary death.
- Cerebrovascular disease, stroke and transient ischemic attack (TIA).
- Peripheral arterial disease, intermittent claudication and significant limb ischemia.
- Aortic disease: Aortic atherosclerosis, thoracic aortic aneurysm, and abdominal aortic aneurysm.
An individual’s risk for future cardiovascular events is modifiable, by lifestyle changes and preventive medical treatment. Lifestyle changes can include stopping smoking, healthy diet, regular exercise, etc. Preventive medical treatment can include a statin, mini dose aspirin, treatment for hypertension, etc. It is important to be able to predict the risk of an individual patient, in order to decide when to initiate lifestyle modification and preventive medical treatment.
Multiple risk models for the prediction of cardiovascular risk of individual patients have been developed. One such key risk model is the Framingham Risk Score.
The Framingham Risk Score is based on findings from the Framingham Heart Study.
Validation[edit]
The Framingham Risk Score has been validated in the USA, both in men and women, both in European Americans and African Americans.[9] While several studies have claimed to improve on the FRS, there is little evidence for any improved prediction beyond the Framingham risk score [10]
Limitations[edit]
There are two limitations.
The Framingham Risk Score predicts only future coronary heart disease (CHD) events, however, it does not predict future total cardiovascular events, meaning that it does not predict risk for stroke, transient ischemic attack (TIA), and heart failure. These also important patient outcomes were included in the 2008 Framingham General Cardiovascular Risk Score.[11] The predicted risk for an individual usually is higher with the 2008 Framingham General Cardiovascular Risk Score than with the 2002 Framingham Risk Score.
The Framingham Risk Score could overestimate (or underestimate) risk in populations other than the US population,[12][13] and within the USA in populations other than European Americans and African Americans, e.g. Hispanic Americans and Native Americans.[14] It is not yet clear if this limitation is real, or appears to be real because of differences in methodology, etc. As a result, other countries may prefer to use another risk score, e.g. SCORE (HeartScore is the interactive version of SCORE - Systematic COronary Risk Evaluation),[15] which has been recommended by the European Society of Cardiology in 2007.[16]
If possible, a cardiology professional should select the risk prediction model which is most appropriate for an individual patient and should remember that this is only an estimate.
Versions[edit]
The current version of the Framingham Risk Score was published in 2008. The publishing body is the ATP III, i.e. the «Adult Treatment Panel III», an expert panel of the National Heart, Lung, and Blood Institute, which is part of the National Institutes of Health (NIH), USA.
The prior version was published in 2002 [17]
The original Framingham Risk Score had been published in 1998.[18]
Differences between the versions[edit]
The first Framingham Risk Score included age, sex, LDL cholesterol, HDL cholesterol, blood pressure (and also whether the patient is treated or not for his/her hypertension), diabetes, and smoking. It estimated the 10-year risk for coronary heart disease (CHD). It performed well, and correctly predicted 10-year risk for CHD in American men and women of European and African descent.
The updated version was modified to include dyslipidemia, age range, hypertension treatment, smoking, and total cholesterol, and it excluded diabetes, because Type 2 diabetes meanwhile was considered to be a CHD Risk Equivalent, having the same 10-year risk as individuals with prior CHD. Patients with Type 1 diabetes were considered separately with slightly less aggressive goals; while at increased risk, no study had shown them to be at equivalent risk for CHD as those with previously diagnosed coronary disease or Type 2 diabetes.[17]
CHD Risk Equivalent[edit]
Some patients without known CHD have risk of cardiovascular events that is comparable to that of patients with established CHD. Cardiology professionals refer to such patients as having a CHD risk equivalent. These patients should be managed as patients with known CHD.
CHD risk equivalents are patients with a 10-year risk for MI or coronary death >20%. CHD risk equivalents are primarily other clinical forms of atherosclerotic disease. The National Cholesterol Education Program NCEP's ATP III guidelines also list diabetes as a CHD risk equivalent since it also has a 10-year risk for CHD around 20%. NCEP ATP III CHD risk equivalents are:
- clinical coronary heart disease (CHD)
- symptomatic carotid artery disease (CAD)
- peripheral arterial disease (PAD)
- abdominal aortic aneurysm (AAA)
Analysis of the US population with the Framingham/ATP III criteria[edit]
The Framingham/ATP III criteria were used to estimate CHD risk in the USA. Data from 11,611 patients from a very large study, the NHANES III, were used. The patients were 20 to 79 years of age, and had no self reported CHD, stroke, peripheral arterial disease, or diabetes.
The results: 82% of patients had low risk (10% or less CHD risk at 10 years). 16% had intermediate risk (10-20%). 3% had high risk (20% or more).[19]
High risk was most commonly found in patients with advanced age, and was more common in men than women.High risk patients are sometimes become unknown with certain signs and symptoms of cardiac failure
Scoring[edit]
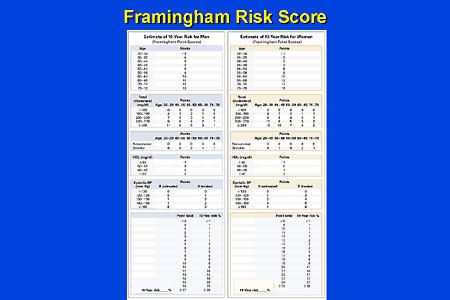
Framingham Risk Score for Women[edit]
Age: 20–34 years: Minus 7 points. 35–39 years: Minus 3 points. 40–44 years: 0 points. 45–49 years: 3 points. 50–54 years: 6 points. 55–59 years: 8 points. 60–64 years: 10 points. 65–69 years: 12 points. 70–74 years: 14 points. 75–79 years: 16 points.
Total cholesterol, mg/dL: Age 20–39 years: Under 160: 0 points. 160-199: 4 points. 200-239: 8 points. 240-279: 11 points. 280 or higher: 13 points. • Age 40–49 years: Under 160: 0 points. 160-199: 3 points. 200-239: 6 points. 240-279: 8 points. 280 or higher: 10 points. • Age 50–59 years: Under 160: 0 points. 160-199: 2 points. 200-239: 4 points. 240-279: 5 points. 280 or higher: 7 points. • Age 60–69 years: Under 160: 0 points. 160-199: 1 point. 200-239: 2 points. 240-279: 3 points. 280 or higher: 4 points. • Age 70–79 years: Under 160: 0 points. 160-199: 1 point. 200-239: 1 point. 240-279: 2 points. 280 or higher: 2 points.
If cigarette smoker: Age 20–39 years: 9 points. • Age 40–49 years: 7 points. • Age 50–59 years: 4 points. • Age 60–69 years: 2 points. • Age 70–79 years: 1 point.
All non smokers: 0 points.
HDL cholesterol, mg/dL: 60 or higher: Minus 1 point. 50-59: 0 points. 40-49: 1 point. Under 40: 2 points.
Systolic blood pressure, mm Hg: Untreated: Under 120: 0 points. 120-129: 1 point. 130-139: 2 points. 140-159: 3 points. 160 or higher: 4 points. • Treated: Under 120: 0 points. 120-129: 3 points. 130-139: 4 points. 140-159: 5 points. 160 or higher: 6 points.
10-year risk in %: Points total: Under 9 points: <1%. 9-12 points: 1%. 13-14 points: 2%. 15 points: 3%. 16 points: 4%. 17 points: 5%. 18 points: 6%. 19 points: 8%. 20 points: 11%. 21=14%, 22=17%, 23=22%, 24=27%, >25= Over 30%
Framingham Risk Score for Men[edit]
Age: 20–34 years: Minus 9 points. 35–39 years: Minus 4 points. 40–44 years: 0 points. 45–49 years: 3 points. 50–54 years: 6 points. 55–59 years: 8 points. 60–64 years: 10 points. 65–69 years: 11 points. 70–74 years: 12 points. 75–79 years: 13 points.
Total cholesterol, mg/dL: Age 20–39 years: Under 160: 0 points. 160-199: 4 points. 200-239: 7 points. 240-279: 9 points. 280 or higher: 11 points. • Age 40–49 years: Under 160: 0 points. 160-199: 3 points. 200-239: 5 points. 240-279: 6 points. 280 or higher: 8 points. • Age 50–59 years: Under 160: 0 points. 160-199: 2 points. 200-239: 3 points. 240-279: 4 points. 280 or higher: 5 points. • Age 60–69 years: Under 160: 0 points. 160-199: 1 point. 200-239: 1 point. 240-279: 2 points. 280 or higher: 3 points. • Age 70–79 years: Under 160: 0 points. 160-199: 0 points. 200-239: 0 points. 240-279: 1 point. 280 or higher: 1 point.
If cigarette smoker: Age 20–39 years: 8 points. • Age 40–49 years: 5 points. • Age 50–59 years: 3 points. • Age 60–69 years: 1 point. • Age 70–79 years: 1 point.
All non smokers: 0 points.
HDL cholesterol, mg/dL: 60 or higher: Minus 1 point. 50-59: 0 points. 40-49: 1 point. Under 40: 2 points.
Systolic blood pressure, mm Hg: Untreated: Under 120: 0 points. 120-129: 0 points. 130-139: 1 point. 140-159: 1 point. 160 or higher: 2 points. • Treated: Under 120: 0 points. 120-129: 1 point. 130-139: 2 points. 140-159: 2 points. 160 or higher: 3 points.
10-year risk in %:Points total:0 point: <1%.1-4 points: 1%.5-6 points: 2%.7 points: 3%.8 points: 4%.9 points: 5%.10 points: 6%.11 points: 8%.12 points: 10%.13 points: 12%.14 points: 16%.15 points: 20%.16 points: 25%.17 points or more: Over 30%.[20]
Further risk score profiles based on the Framingham Heart Study[edit]
Not only coronary heart disease (CHD) events, but also further risks can be predicted. Risk prediction models for cardiovascular disease outcomes other than CHD events have also been developed by the Framingham Heart Study researchers. Amongst others, a risk score for 10-year risk for atrial fibrillation has been developed.[21][22]
A calculator for 10-year risk for atrial fibrillation is available on the Framingham Heart Study website: https://www.framinghamheartstudy.org/fhs-risk-functions/atrial-fibrillation-10-year-risk/
See also[edit]
- Framingham Risk Score Calculator for Coronary Heart Disease - Cholesterol in mg/dL
- Framingham Coronary Heart Disease Risk Score - Cholesterol in mg/dL or mmol/L
References[edit]
- ^P.W., Wilson; D'Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. (12 May 1998). 'Prediction of coronary heart disease using risk factor categories'. Circulation. 97 (18): 1837–1847. doi:10.1161/01.CIR.97.18.1837. PMID9603539. Retrieved 7 May 2013.
- ^D'Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. (22 January 2008). 'General cardiovascular risk profile for use in primary care: the Framingham Heart Study'. Circulation. 117 (6): 743–753. doi:10.1161/circulationaha.107.699579. PMID18212285.
- ^Collins, Dylan; Lee, Joseph; Bobrovitz, Niklas; Koshiaris, Constantinos; Ward, Alison; Heneghan, Carl (2016-10-14). 'Simple and adaptable R implementation of WHO/ISH cardiovascular risk charts for all epidemiological subregions of the world'. F1000Research. 5: 2522. doi:10.12688/f1000research.9742.1.
- ^'Cardiovascular Risk Calculator and Chart v3.0'. Cvrisk.mvm.ed.ac.uk. 2010-05-19. Retrieved 2013-09-14.
- ^'Risk Scoring Systems'. Framingham Heart Study. Retrieved 7 May 2013.
- ^Wilson, PW; D'Agostino, RB; Levy, D; Belanger, AM; Silbershatz, H; Kannel, WB (1998). 'Prediction of coronary heart disease using risk factor categories'. Circulation. 97 (18): 1837–47. doi:10.1161/01.cir.97.18.1837. PMID9603539.
- ^Estimation of cardiovascular risk in an individual patient without known cardiovascular disease. Wilson PWF. In: UpToDate [Textbook of Medicine]. Basow, DS (Ed). Massachusetts Medical Society, and Wolters Kluwer publishers, The Netherlands. 2010.
- ^Collins, Dylan Raymond James; Tompson, Alice; Onakpoya, Igho; Roberts, Nia; Ward, Alison; Heneghan, Carl (2017). 'Global cardiovascular risk assessment in the primary prevention of cardiovascular disease in adults: systematic review of systematic reviews'. BMJ Open. 7 (3): e013650. doi:10.1136/bmjopen-2016-013650. PMC5372072. PMID28341688.
- ^Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. JAMA. 2001 Jul 11;286(2):180-7.
- ^Tzoulaki, I.; Liberopoulos, G.; Ioannidis, JP. (Dec 2009). 'Assessment of claims of improved prediction beyond the Framingham risk score'. JAMA. 302 (21): 2345–52. doi:10.1001/jama.2009.1757. PMID19952321.
- ^D'Agostino RB Sr; Vasan RS; Pencina MJ; Wolf PA; Cobain M; Massaro JM; Kannel WB (Feb 2008). 'General cardiovascular risk profile for use in primary care: the Framingham Heart Study'. Circulation. 117 (6): 743–53. doi:10.1161/circulationaha.107.699579. PMID18212285.
- ^Brindle P, Emberson J, Lampe F, Walker M, Whincup P, Fahey T, Ebrahim S (Nov 2003). 'Predictive accuracy of the Framingham coronary risk score in British men: prospective cohort study'. BMJ. 327 (7426): 1267. doi:10.1136/bmj.327.7426.1267. PMC286248. PMID14644971.
- ^Liu J; Hong Y; D'Agostino RB Sr; Wu Z; Wang W; Sun J; Wilson PW; Kannel WB; Zhao D (Jun 2004). 'Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study'. JAMA. 291 (21): 2591–9. doi:10.1001/jama.291.21.2591. PMID15173150.
- ^Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC (Dec 2009). 'Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study)'. J Am Coll Cardiol. 54 (24): 2303–11. doi:10.1016/j.jacc.2009.07.047. PMID19958966.
- ^Conroy R (2003). 'Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project'. European Heart Journal. 24 (11): 987–1003. doi:10.1016/s0195-668x(03)00114-3.
- ^Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM (Jun 2003). 'Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project'. Eur Heart J. 24 (11): 987–1003. doi:10.1016/s0195-668x(03)00114-3.
- ^ abThird Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143-421.
- ^Prediction of coronary heart disease using risk factor categories. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Circulation. 1998 May 12;97(18):1837-47.
- ^The distribution of 10-Year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. Ford ES, Giles WH, Mokdad AH. J Am Coll Cardiol. 2004 May 19;43(10):1791-6.
- ^'NHLBI, Estimate of 10-Year Risk for CHD'. Nhlbi.nih.gov. Retrieved 2013-09-14.
- ^Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton-Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Lancet. 2009 Feb 28;373(9665):739-45.
- ^Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Circulation. 2004 Aug 31;110(9):1042-6.